Controlled-release pharmaceuticals
日本語
English
PLGA™
Sustained release pharmaceutical base material
Applications detail
Applications
- Overview
- Properties
Overview
Mitsui PLGA is a high-quality excipient for the controlled-extended release of drugs for industrial usage.
Properties
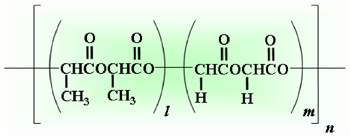
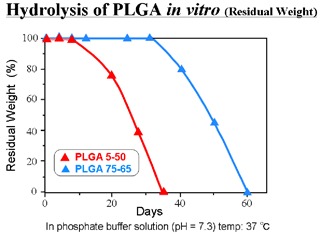
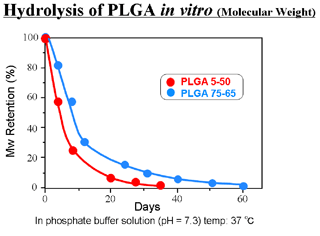
- Degraded into glycolic acid and lactic acid by hydrolysis
- Specific rate of degradation depending on its copolymer ratio and molecular weight
Physical Properties
| CAS No. | 26780-50-7 |
|---|---|
| Chemical Formula | ( C4H4O4 )x ( C6H8O4 )y |
| Color | white to light tan |
| Odor | odorless |
| Tm (Melting Point) / ℃ | - |
| Tg (Glass Transition) / ℃ | 45-55 |
| Solvent | CH2Cl2 CHCl3 DMF DMSO THF |
Properties of the Standard Grade
| Polymer | Co-polymer Ratio | *Inherent Viscosity | **Molecular Weight | Residual Monomer | Sn Content | Heavy Metals | Sulphated Ash | ||
|---|---|---|---|---|---|---|---|---|---|
| (mol%) | (dL/g) | (kDa) | (%) | (ppm) | (ppm) | (%) | |||
| GLD | LTD | GLD | LTD | ||||||
| PLGA 5-50 | 45-55 | 45-55 | 0.45-0.60 | (50-55) | < 2.0 | < 2.0 | < 50 | < 10 | < 0.1 |
| PLGA 75-75 | 20-30 | 70-80 | 0.72 – 0.80 | (78 – 85) | < 2.0 | < 2.0 | < 50 | < 10 | < 0.1 |
| PLGA 75-65 | 0.60-0.70 | (62-76) | |||||||
| PLGA 75-50 | 0.45-0.55 | (50-60) | |||||||
- Mitsui PLGA GRADE NAMING
- PLGA Lactide-Viscosity
- EX : PLGA 5-50
- Lactide 50 : 50, Viscosity 0.50 dL/g
*Inherent Viscosity : 0.5 g/dl (Chloroform), @25 C
**Molecular Weight (by GPC) : (Standard polymer-Solvent) PMMA-HFIP for PGA, PS-THF for PLGA
We also welcome your requests for customized products, with a minimum quantity of 5 kg/year.
Contact Us
Personal Care Materials Division
TEL
+81-3-6880-7451
FAX
+81-3-6880-7561

