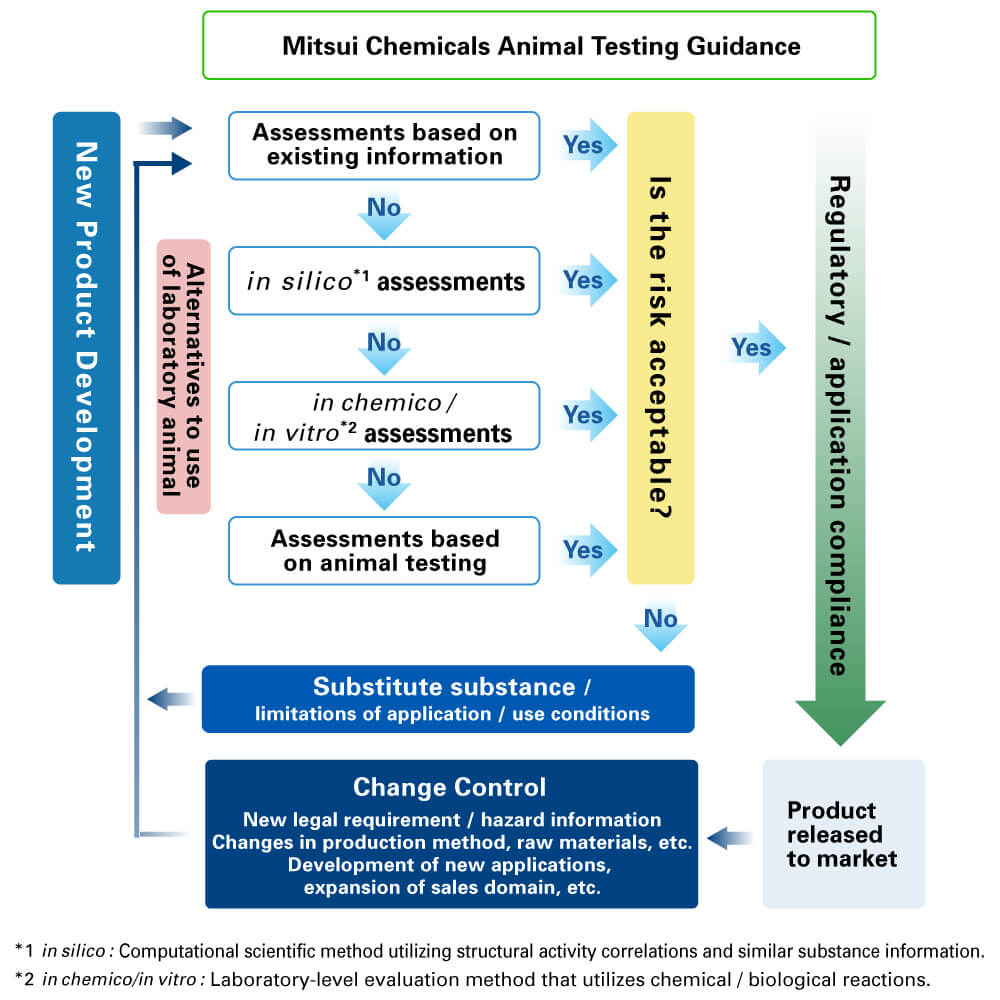
For human health and environmental protection, as well as for sustainable development, the Sound Chemicals and Waste Management scheme for chemical substances and waste, which takes into account product life cycles, is being proposed and is also being deployed by the International Council of Chemical Associations (ICCA). In addition, the Global Framework on Chemicals (GFC) for 2030 calls for the promotion of voluntary chemical substances management by multiple stakeholders, including industry.
As a member of the supply chain that aims for sustainable development, the Mitsui Chemicals Group is engaging in business and product development that incorporates those sound and voluntary perspectives on chemical substances management.